The NIH Clinical Center is the world's largest hospital entirely devoted to clinical research. It is a national resource that makes it possible to rapidly translate scientific observations and laboratory discoveries into new approaches for diagnosing, treating, and preventing disease.
Clinical research is at the heart of the Clinical Center's mission.
Over 1,600 clinical research studies are conducted at the NIH Clinical Center, including those focused on cancer, infectious diseases, blood disorders, heart disease, lung disease, alcoholism and drug abuse. Most of these studies are sponsored by the Institutes and Centers at NIH.
Here is a sample of abstracts from the clinical research conducted at the NIH Clinical Center and published in a peer reviewed medical journal this year. Links to the full text and video formats are provided if available.
2026
Disease-causing STAT3 variants can be discriminated by a functional flow cytometry test
Published in: Pediatric Allergy and Immunology (February 2026)
Mutations in the STAT3 gene can result in increased or decreased function, affecting cell growth and immune system responses, which cause human diseases. However, STAT3-mutation classification is a bottleneck for patient diagnosis and treatment. Researchers report they have optimized a single flow cytometry-based functional test able to determine the function of STAT3 mutations. The test is adapted for clinical laboratories, enabling accessible and reliable evaluation of STAT3 genetic changes.
Read the article.

Associations Between Fatty Acid Levels in Human Blood and Trigeminovascular Tissues
Published in: Lipids (January 2026)
Omega-3 and omega-6 polyunsaturated fatty acids (PUFAs) are precursors to oxylipins that modulate pain and inflammation. Study authors previously found that dietary intervention increasing omega-3 and reducing omega-6 PUFAs alters the concentration of these oxylipin precursors in blood, changes associated with reduced headache pain. They now report that blood measurements of certain PUFAs can serve as a proxy of their concentration in tissues directly involved in headache onset.
Read the article.
2025
Systematic Functional Validation of IKAROS Variants From Patients and Laboratory-Generated Mutations
Published in: Blood Advances (December 2025)
Mutations in the IKAROS gene, which controls immune cell development, can lead to serious health issues, such as leukemia or immune system failure. In a new study, researchers recreated genetic “typos” in the lab to see how they affected the protein’s job, testing 33 variants found in patients and 48 lab-made mutations. Of the 81 genetic changes tested, 36 were found to be harmful. An accompanying map tells doctors if specific mutations are more or less likely to cause disease.
Read the article.

Peppers and Poppies: A Perspective on Nociceptors and Pain Control
Published in: Pain (December 2025)
Clinical Center researchers outline a strategy for developing new non-opioid analgesics, avoiding the shortcomings of opioid-based pain treatments, such as morphine and fentanyl, which carry many bad side effects and can lead to addiction. Authors of the topical review say new approaches to develop new analgesics are needed.
Read the article.
Dual-Camera Augmented Reality-Based Navigation System for Precision CT-Guided Biopsy and Ablation
Published in: Cardiovascular Interventional Radiology (December 2025)
Researchers tested an experimental navigation system using two cameras mounted on a CT scanner to help doctors guide needles during procedures like biopsies. Overlaying virtual maps of a patient’s anatomy onto live video, the system was significantly more accurate and twice as fast as the traditional “freehand” method.
Read the article.
Dementia Intervention Trials and Opportunity Costs for Potential Participants
Published in: Alzheimer Disease and Associated Disorders (December 2025)
For persons with or at risk for Alzheimer's disease (or other dementias), the paucity of treatment options and lengthy clinical trials carry an opportunity cost: choosing to participate in one study means foregoing others. The authors of a new paper say there are strong ethical reasons for scientists to disclose alternate research options to potential study participants prior to their enrollment, and suggest some efficient, non-burdensome ways to do so.
Read the article.
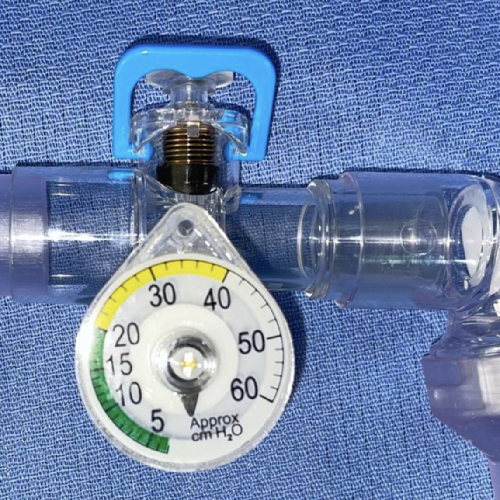
In-line miniature 3D-printed pressure-cycled ventilator
Published in: Anesthesiology (November 2025)
The COVID-19 pandemic demonstrated the need for large quantities of simple, cost-effective, and rapidly scalable ventilator alternatives during a time of extraordinary demand. Using fluidic design principles and 3D-printing technology, researchers developed a miniature, pressure-cycled automated ventilator with no moving parts. Sixteen of the investigational devices can be printed in two hours.
Read the article.
Stability Testing of HPCs and MNCs From Apheresis Products
Published in: Immunobiology (November 2025)
Hematopoietic progenitor cells (HPCs) and mononuclear cells (MNCs) are critical components of cell-based therapies, including bone marrow transplantation and regenerative treatments. In a new study, researchers evaluated the functional and molecular stability of samples collected for the evaluation of fresh HPC and MNC products. They report RNA-sequencing data reveal early transcriptomic changes in HPC samples, suggesting immediate evaluation is critical to preserve their molecular integrity and functionality.
Read the article.

Reflections on a Principal of Research Ethics: Tom Beauchamp, Moral Specification, and Waivers of Informed Consent
Published in: American Journal of Bioethics (October 2025)
Research ethics pioneer Tom Beauchamp articulated key ethical principles central to understanding research ethics scholarship and practice. Authors of a new paper apply Beauchamp’s analytical framework to explore a contemporary ethics challenge: When can the requirements of informed consent permissibly be relaxed for research studies embedded into clinical care?
Read the article.

All You Need to Know About Equipment Validation for Sterility Testing
Published in: Journal of Clinical Microbiology (September 2025)
Product sterility testing requests are becoming increasingly common in clinical microbiology laboratories due to the rapid development of advanced therapies. In a mini-review article, authors provide an overview of the four-phase IOPQ framework (Installation, Operational, and Performance Qualification), what is included and how it differs from CAP requirements, important considerations for an IOPQ, and a summary of FDA citations relating to equipment validation.
Read the article.

Alcohol, Aging, and the Gut Microbiome: Intersections of Immunity, Barrier Dysfunction, and Disease
Published in: Alcohol (July 2025)
In a review article, researchers synthesize the most up-to-date understanding of how alcohol, host genetics, the gut microbiome, immune regulatory pathways and aging intersect to influence disease risk. The authors argue that future research should prioritize precision-based, gut-targeted strategies aimed at restoring microbial balance, maintaining intestinal barrier integrity and mitigating alcohol-related harm across the lifespan.
Read the article.

Hypertension and the Gut Microbiome: A Science Advisory From the American Heart Association
Published in: Hypertension (July 2025)
Numerous preclinical studies show that alterations in the gut microbiome can affect blood pressure. The association presents a novel therapeutic opportunity for hypertension: modifying the gut microbiome to control hypertension, the authors of a detailed new American Heart Association Science Advisory write.
Read the article.
Transitioning a multiethnic donor pool from serologic D negative to molecularly RHD negative at a hospital based blood donor service
Published in: Journal of Translational Medicine (June 2025)
Some individuals carry a very low expression of the D-antigen on their red blood cells, known as the Del phenotype. Such cells are RhD protein-positive, despite being routinely labelled D-negative. In a new study, researchers report that molecular typing of blood donors offers a more sensitive method to identify Del individuals by detecting the presence of the RHD gene and enhances transfusion safety for patients.
Read the article.

Assessing the Impact of Cell Isolation Method on B Cell Gene Expression Using Next-Generation Sequencing
Published in: Experimental Hematology (June 2025)
Despite widespread use of immune cell type-specific isolation methods for human B cells, little research has been done on their effect on genetic transcription. New research reports significant gene expression changes following CD19-dependent B cell isolation via positive magnetic cell sorting (MCS) or fluorescence-activated sorting compared to negative MCS. The findings bear consideration during experiment design and sequencing dataset analysis, study authors say.
Read the article.
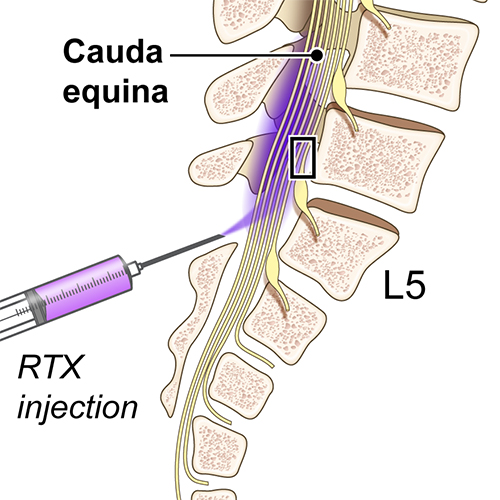
Treatment of Intractable Cancer Pain with Resiniferatoxin — An Interim Study
Published in: NEJM Evidence (May 2025)
A first-in-human clinical trial of a new therapy based on the plant-derived molecule resiniferatoxin (RTX) shows that it is safe and effective for control of intractable pain in patients with advanced cancer. Researchers found that a single injection of small quantities of RTX by lumbar puncture into the cerebrospinal fluid of cancer patients reduced their reported worst pain intensity by 38% and their use of pain-relieving opioids by 57%.
Read the article.
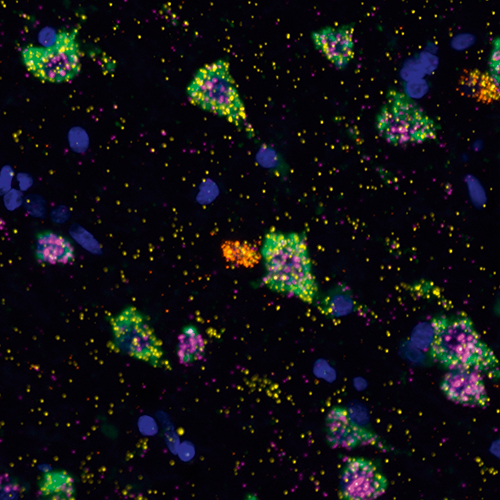
Efficient Removal of Naturally Occurring Lipofuscin Autofluorescence in Human Nervous Tissue Using High-Intensity White Light
Published in: The Journal of Pain (May 2025)
Lipofuscin is a naturally occurring pigment present in neurons of dorsal root ganglia and central nervous system that interferes with microscopy techniques used to study healthy and diseased human nervous system tissues. In a new study, researchers describe a low-cost procedure that can be effectively remove interfering signals and provide much clearer fluorescent microscopy images to enable high-plex, direct-in-human research.
Read the article.

Differences in Care and Outcomes in Cardiogenic Shock in Cardiac Intensive Care Units in the United States and Canada: CCCTN Registry Insights
Published in: Canadian Journal of Cardiology (April 2025)
Despite the evolution of cardiogenic shock (CS) teams, in-hospital mortality for CS patients remains high and high-quality evidence to guide optimal treatment remains scarce. In a new study, researchers analyzed data from 34 American and 8 Canadian tertiary cardiac intensive care units gathered from 2017 to 2022. The authors describe differences in practices and CS patient clinical outcomes.
Read the article.

Do Caregivers Value the New Antiamyloid Treatments for Alzheimer's Disease More Than Home-Based Care?
Published in: The American Journal of Geriatric Psychiatry (April 2025)
While the new FDA-approved drugs for Alzheimer’s disease mark a significant advancement, this study surveyed family caregivers’ views on the value of the new drugs by comparing them to home-based care. Though caregivers indicated a desire for their loved one to receive both medication and home-based care, just over half (57%) preferred insurance coverage of home-based care. Those who favored coverage of home-based care were more likely to think that the medication’s benefits do not outweigh its risks and burdens.
Read the article.
Assessing the Reliability of Pancreatic CT Imaging Biomarkers for Diabetes Prediction: A Dual Center Retrospective Study
Published in: Academic Radiology (March 2025)
In a retrospective study, researchers analyzed the CT scans and HbA1c average blood sugar tests of 9,772 patients with and without diabetes from two institutions. Among other findings, the study suggests CT-derived imaging biomarkers, or disease indicators, could be a reliable tool for diabetes screening across multiple institutions.
Read the article.

The Relationship Between Stiff Knee Gait Runner's Dystonia and Musculoskeletal Knee Pathology: A Case Series
Published in: Toxins (March 2025)
Runner's Dystonia (RD) is a rare movement disorder causing involuntary muscle contractions in the lower limbs during running. This condition is often overlooked or misdiagnosed due to RD patients overlapping symptomology with other conditions. Treatments to address neurological and musculoskeletal factors could improve patient outcomes. This research paves the way for personalized treatment plans and brings RD awareness to prevent long-term disability and improve quality of life.
Read the article.

Epidemiology and Prognostic Significance of Acute Noncardiac Organ Dysfunction Across Cardiogenic Shock Subtypes
Published in: Journal of Cardiac Failure (February 2025)
Researchers analyzed medical data on 3,904 admissions for cardiogenic shock (CS) from 2017–2022 across 42 facilities. Their goal was to better understand the patterns and prognostic clues of acute noncardiac organ dysfunction across subtypes of CS. They found admissions for acute-on-chronic heart failure (HF)-CS had a lower burden of acute noncardiac organ dysfunction compared with admissions for de novo HF-CS and acute myocardial infarction (AMI) related CS. The study authors say their findings may help further improve risk stratification and prognostication in patients admitted with CS related to AMI, de novo HF, and acute-on-chronic HF.
Read the article.
Read more articles about research in the NIH Clinical Center in 2025.

