|

Published
monthly for CC employees by Clinical Center Communications
past issues
August
2000
Register now for new clinical research training program
Decker, former CC director, dies July 13
Quality of Worklife Committee reports on CC suggestions
Food now available Lickity Split
Education programs in clinical research growing
Medicine for the Public begins next month
Exhibit spotlighting strength from unity runs through Sept. 4
Caring
for the animals
News
briefs
|
|
 Register
now for new clinical research training program Register
now for new clinical research training program
A new course for clinical
principal investigators, "Clinical Research Training," will be
offered Sept. 12 from noon to 4 p.m. in Lipsett Amphitheater. This course
was designed to address one of the essential standards recently approved
by the NIH for performing clinical research. All clinical principal investigators
are required to take the course and successfully complete a final exam.
Former participants
of the "Introduction to the Principles and Practice of Clinical Research"
and "Ethical and Regulatory Aspects of Human Subjects Research"
who have passed both courses, as exhibited by successful completion of
the final exam given in each program, will not be required to take the
course.
Course topics:
- Historical and Ethical
Perspectives, Dr. Ezekiel J. Emanuel, chief, Department of Clinical
Bioethics, CC.
- Roles and Responsibilities
of the Investigator, Dr. Gregory Curt, clinical director, NCI.
- Roles and Responsibilities
of the Institution, Dr. Alison Wichman, deputy director, Office of Human
Subjects Research, OD.
- Regulatory Issues,
Dr. Jay Siegel, director, Therapeutics Research and Review, FDA Center
for Biologics Evaluation and Research.
- Clinical Investigators
and the Mass Media, Anne Thomas, associate director for communications,
OD.
Enrollment in the initial
course offering will be limited to those individuals for whom it is required.
Registration, which is online, ends Aug. 30. The course will be repeated Dec. 12.
|
 Decker,
former CC director, dies July 13 Decker,
former CC director, dies July 13
Dr. John Laws Decker, Clinical
Center director and NIH Associate Director for Clinical Care from 1983 until
his retirement in 1990, died of a heart arrythmia July 13 in Bethesda.
During his retirement, Dr.
Decker remained active at the Clinical Center, serving as author, and in later
editions contributing editor, of "Protomechanics, A Guide to Preparing
and Conducting a Clinical Research Study." He also was a consultant to
the FDA.
Dr. Decker steered the Clinical
Center through challenging times. He said in a 1990 interview, "The most
challenging aspect of my years here has been trying to do all that I could to
accelerate the changes required by Congress in reference to research on AIDS.
It was a brand new disease when I took over the directorship." Major advances
at the CC during Dr. Decker's tenure included development of the PET program
and clinical use of the MRI.
Dr. Decker came to NIH in
1965 as a chief of the Arthritis and Rheumatism Branch in what is now the National
Institute of Arthritis and Musculoskeletal and Skin Diseases, serving as clinical
director from 1976-1980 and scientist emeritis following his retirement in 1990.
A native of New York and the son of missionary parents, he grew up in China
and returned to the U.S. for his education. A World War II Navy veteran, Dr.
Decker served in the Pacific and received the Purple Heart.
A graduate of the University
of Richmond, he earned his MD from Columbia University College of Physicians
and Surgeons in 1951. From 1951-1955, he completed his internship and residency
requirements at Presbyterian Hospital in New York. He went on as a research
fellow in medicine at Harvard University and at Massachusetts General Hospital,
where he received training in rheumatology. Before coming to NIH, Dr. Decker
was on the faculty at the University of Washington in Seattle.
His studies in rheumatic
diseases earned him international recognition. Among his awards: the Philip
Hench Award from the Association of Military Surgeons in 1972, the NIH Director's
Award in 1977, the Alessandro Robecchi International Prize for Rheumatology
Research on nephritis of systemic lupus erythematosus in 1983, and the PHS Superior
Service Award in 1987. Dr. Decker in 1989 became the second physician to receive
the American College of Rheumatology Gold Medal. In 1990, Dr. Decker was the
first NIH physician to receive the Master of the American College of Physicians.
Survivors include his wife,
Lucille Macbeth Decker of Bethesda; son David L. of Bethesda; and three daughters,
Virginia E. Jahnes of Jefferson, Md., Margaret "Megan" Malaro of Chestertown,
Md., and Susan Morrow of Bristol, Va.
Quality
of Worklife Committee reports on CC suggestions
|
|
The
CC QWL Council appreciates your suggestions and input. If you have any suggestions
and/or proposed solutions, please visit the CC QWL suggestion box near the
B1 and 2nd floor cafeteria exits. |
The Clinical Center Quality
of Worklife Committee recently received the following suggestions:
Suggestion: Ladies'
restrooms are dirty and "unsafe," which is not always the fault of
the housekeeping staff. Can NIH place signs asking users to clean up after themselves
and wash their hands?
Response: This suggestion
was forwarded to the Housekeeping and Fabric Care Department management for
input. We were informed that there are signs in the restrooms throughout the
building advising people who to call if the restroom is not up to par. The men's
rooms in the east half of the building all have "Please Flush" signs
over the urinals, but it has not made much difference. A study is currently
under way to see if signage helps. All bathrooms are cleaned daily, and the
most used ones are cleaned several times a day.
One major problem is that
we have more restroom users than restrooms, but this should be better when the
CRC is complete, as more restrooms are slated to be built. Last year, the CC
Quality of Worklife Committee distributed a desk-to-desk "Spring Cleaning"
factsheet with some helpful hints for staff.Visit http://www.cc.nih.gov/ccc/qwi/spring.html
for more information.
Suggestion: The stamp
machine does not work. Can NIH get its own post office? Response: Our
CC and ORS research indicates that plans are currently under way for a full-service
post office, which would also provide copying, faxing, and other services for
employees and visitors. However, the new post office will be established as
part of the Building 10 revitalization and establishment of the CRC in 2002.
In the meantime, ORS staff
indicate that they are discussing interim plans for improving or upgrading current
post office services in Building 10. Unfortunately, there really isn't space
for anything more elaborate than what currently exists in the B1 basement station.
With regard to the specific problem of the stamp machines being broken, we learned
that the Bethesda postal station, the service provider, is in transition and
has been short-staffed. A new permanent staff person will be assigned to Building
10 within three weeks. That individual will become the Building 10 point-person
for faulty stamp machines and related problems.
In case you are not aware,
the Recreation and Welfare Association (R&W;) also sells stamps. The council
will continue exploring other possibilities for improving the postal service.
Food
now available Lickity Split
Work nights? Need a meal
when the cafeteria's closed?
Eurest has developed a new
program that will bring the food to you. This service, available only in Building
10, is called "Lickity Split." It's offered to provide a meal or snack
for those whose schedules keep them busy while the cafeteria is open. Stop by
the 2nd floor cafeteria anytime during the day and pick up an order form. The
form lists the items available with options for you to pick, including wrap
sandwiches, salads, cookies, and beverages. Circle the items you would like
and take it to the cashier. Make sure to put your name and delivery location
in the proper place on the form and pay the cashier. When the cafeteria closes--9
p.m. during the week and 6 p.m. weekends--Eurest will deliver your order. The
package will be sealed and a copy of the order form attached. Call 6-9698 for
more information.
|

Duke
Grads
Spring
graduates of the NIH-Duke Training Program in Clinical Research
included Richard Messman, left, and Stefan Weiss. Not pictured are
Michael Brennan, Hiroyu Hatano, and Kara Sovik. The program--which
began in 1998--is a collaboration between the Clinical Center and
Duke University, in Durham, N.C. It uses distance- learning to strengthen
training opportunities in clinical research. The Duke University
School of Medicine, which established its program in 1986, awards
a Master of Health Sciences in Clinical Research for successful
completion of the training program here.
|
|

Fellows
arrive
A reception
following orientation for this year's incoming clinical fellows
provided an opportunity for new and current trainees to meet one
another, as well as chat informally with NIH senior staff and others
who will be vital in their training experience. Standing left is
Dr. John I. Gallin, CC director.
|
|
Education
programs in clinical research growing
Clinical research--evaluating
new and promising treatments in people--provides a blueprint for better
health care. To help refine and focus those blueprints, the CC has developed
a series of programs aimed at improving how clinical research is conceived,
monitored, and carried out.
"This is an extraordinary
era of innovation and progress for medicine and science," notes Dr.
John I. Gallin, CC director. "Clinical research can be beneficial
and successful only when physician-researchers have the necessary training
and expertise to conduct it. Historically, medical students depended on
willing and able mentors to teach the intricacies of clinical research.
That approach simply doesn't work today."
The nation's clinical
research hospital, the NIH Clinical Center offers an excellent environment
for exploring new approaches to identifying and providing the tools that
researchers need. "Effective clinical research is both an art and
a science," Dr. Gallin adds, "and training in clinical research
depends on a thorough grounding in the basic techniques, rich opportunities
for practical application, and the flexibility to meet the changing needs
of medical science and health-care consumers."
How do clinical
researchers learn? Bridging the gap.
Cornerstone for Clinical
Center training efforts is the course, "Introduction
to the Principles and Practice of Clinical Research," established
in 1995. "The program teaches researchers how to design a good clinical
trial," Dr. Gallin, who serves as course director, points out. It
covers epidemiological methods and focuses on study design and development,
protocol preparation, patient monitoring, quality assurance, and FDA issues.
It also includes data
management and legal and ethical issues, including protection of human
subjects. The course is offered annually. Classes meet twice a week September-February.
Some attend by teleconference sites in Baltimore, Georgetown University
and the University of Puerto Rico. The course also has been video-conferenced
to NIEHS in North Carolina, NIDDK components in Arizona, and to the Rocky
Mountain Labs in Montana, which are part of NIAID.
Another distance-learning
program designed to strengthen training opportunities in clinical research
is a collaboration
between the NIH Clinical Center and Duke University that began in
1998. It's designed primarily for clinical fellows and other health professionals
training for careers in clinical research. "It meets an existing
need at NIH for formalized academic training in the quantitative and methodological
principles for clinical research," Dr. Gallin says. Offered are courses
in research design, statistical analysis, health economics, research ethics,
and research management. NIH participants complete coursework primarily
through videoconferences with faculty at Duke. NIH staff teach other courses
as Duke adjunct faculty.
The Duke University
School of Medicine, which established its program in 1986, awards a Master
of Health Sciences in Clinical Research for successful completion. The
program can be completed in two 16-week semesters. Participants typically
spread course work over two years. There's also a non-degree option for
qualified individuals who want to acquire specific skills in this discipline.
Clinical Pharmacology:
A critical link
Another NIH Clinical
Center-based training opportunity is the course, "Principles
of Clinical Pharmcology," initiated in 1998 by Dr. Art Atkinson.
"Many medical schools don't offer formal courses in clinical pharmacology,"
Dr. Gallin says. "This program covers what researchers need to know
concerning the clinical pharmacologic aspects of drug development and
use."
The course, which complements
the Introduction to the "Principles and Practice of Clinical Research,"
includes a review of pharmacokinetics, drug metabolism and transport,
assessment of drug effects, drug therapy in special populations, and contemporary
drug development. Offered annually, the course is also designed to assist
individuals preparing to take the certification exams of the American
Board of Clinical Pharmacology.
"A great deal
of the clinical research done here at the Clinical Center involves clinical
trials of new drugs," explained course director Dr. Atkinson. "Clinical
pharmacology is an obviously important component of the design, conduct
and interpretation of these trials and it appears that our course fills
an unmet need in this regard."
Examining ethical
issues
"Medicine and
science can't advance unless society trusts that progress never compromises
the health and well-being of those who participate in clinical research,"
explains Dr. Gallin. "Thoughtful and continuing assessments of ethical
issues are critical skills that clinical researchers need."
To help meet that need,
the Clinical Center's Department of Clinical Bioethics has put together
a seven-session overview of ethical and regulatory issues in clinical
research for NIH intramural scientists and research staff.
"The goal of
the course is to provide clinical investigators and institutional review
board members skills with which they can analyze the ethical issues they
confront in clinical research," said Dr. Ezekiel J. Emanuel, course
director and chief of the CC Department of Clinical Bioethics. "We
want to train researchers so they can design their protocols to conform
to prevailing ethical standards on ethical research." The program,
which began in 1998, includes sessions on the history of human-subjects
research; research principles and guidelines; the ethics of clinical trial
design, patient recruitment; and informed consent. A mock IRB (institutional
review board) is incorporated, along with extensive panel discussions,
including presentations from research patients on their experiences.
<
|
 Medicine
for the Public begins next month Medicine
for the Public begins next month
The Medicine for the
Public lecture series begins Sept. 19.
The lectures are held
at 7 p.m. on Tuesdays in the Clinical Center's Masur Auditorium.
The schedule: Sept.
19, New Directions for Organ and Tissue Transplantation, Dr. Allan Kirk,
NIDDK. Sept. 26, Adolescents and AIDS: Millennium Milestones, Dr. Lauren
Wood, NCI. Oct. 3, Dangerous Liaisons: Drugs and Herbal Products, Dr.
Stephen Piscitelli and Dr. Aaron Burstein, CC. Oct. 10, Stroke: Rapid
Diagnosis, New Treatments, Dr. Alison Baird, NINDS. Oct. 17, Women's Health
Research for the 21st Century, Dr. Vivian Pinn, Office of Research on
Women's Health. Oct. 24, Prostate Cancer, Dr. Marston Linehan and Dr.
William Dahut, NCI. Visit the web at http://www.
cc.nih.gov/ccc/mfp/series.html for details.
 Presentations Presentations
Dennis Martell, RN,
research case manager on the 8th floor AIDS Outpatient Clinic, discussed
research he is involved in with a group of visiting faculty from some
of the major nursing schools in Puerto Rico. The NIH Offices of Loan Repayment
and Scholarship and Equal Opportunity and the CC Nursing Department sponsored
two days of presentations with a focus on opportunities in nursing for
faculty and students.
Exhibit
spotlighting strength from unity runs through Sept. 4
Strength from Unity:
Expressions on Cancer from the Island of Ireland and the United States
will be on display in the Lipsett Gallery through Sept. 4. The works were
originallydisplayed in 1999 in Belfast, Northern Ireland, as a part of
the NCI All Ireland Cancer Conference.
Funded by the Susan
G. Komen Breast Cancer Foundation, this is a collection of paintings,
photographs, tapestries, and poems of artists from Northern Ireland, the
Republic of Ireland, and the United States whose lives have been touched
by all types of cancer. Each of the 18 pieces of art in the CC exhibit
is accompanied by the artist's personal story of how he or she was compelled
to work on issues of life, death, strength and above all, hope, through
artistic expression.
Collectively, the
exhibit reflects the idea that cancer knows no political or geographic
boundaries and that there is strength in unity. Bernard Fallon's "Hope"
depicts his wife, Trudy's, cancer diagnosis in 1995. For her, the door
"exudes comfort, light, and strength." That light, she says,
"kept me strong and connected to life through some of the bleakest
days of my illness. I'm here. I'm living."
|
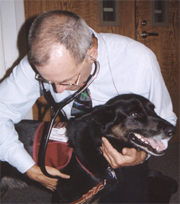
Suchie, a six-year-old
black lab mix, is a member of the Caring Canines team popular with patients
and staff alike at the Clinical Center. Under the organizational umbrella
of National Capital Therapy Dogs, Inc., the dogs visit here four times a
month. Dr. Haines makes sure they are freshly bathed, clipped and cleaned--and
healthy--each time. (Photos by Bonnie Flock) |
 Caring Caring
. . . for the animals
They visit and live
and support research here--a cadre of animals that play an assortment
of roles. Caring Canines are regulars, as are the guinea pigs in the playroom
and the fish that circle in lobby aquariums.
Making sure that all
the animals at the Clinical Center are healthy and well cared for is part
of veterinarian Dr. Mark C. Haines's job. He's the CC's Animal Program
Director. These photos offer a glimpse into the sometimes-unseen aspects
of animal care here.

Accessing and advising
on the conduct of research involving animals is a major aspect of Dr.
Haines' work at the CC. In addition to monitoring the health and safety
of research animals, Dr. Haines consults with investigators writing research
protocols to make sure all animal issues are appropriately addressed.
|
|

Patients enjoy the
calm and color aquariums offered in some Clinical Center waiting areas.
Dr. Haines provides oversight to those who care for the fish.
|
News
briefs
 DTM
symposium set DTM
symposium set
The CC Department of Transfusion
Medicine (DTM), in conjunction with the American Red Cross and Nexell Therapeutics,
Inc., will sponsor the 19th Annual Immuno-hematology & Blood Transfusion Symposium
on September 14 in Masur Auditorium. This all-day conference will include speakers
from the CC, as well as experts in the field from across the nation discussing
molecular biology, blood donors, and transplantation and immune therapy. Dr.
Harvey Klein, DTM chief, will present "Leukoreduction: Boon or Boondoggle."
Dr. Harvey Alter, infectious diseases chief, will discuss "Can You Fool
Mother Nature? A Millennial Perspective on the Natural History of Hepatitis
C." Program and registration details are online:
http://www.cc.nih.gov/dtm.
Task force lauded for best
practices
The CC Chemotherapy Errors
Prevention Task Force received one of six American Society of Health Pharmacists
Best Practices in Health-System Pharmacy Management Awards for their submission,
"An Interdisciplinary Continuous Improvement Approach to Reducing Chemotherapy-Related
Medication Errors." This award honors innovation and outstanding leadership
in health-system pharmacy practice management and pharmaceutical care. Members
of this task forceincluded Charles Daniels, PhD, Robert Dechristoforo, MS, Barry
Goldspiel, PharmD, David Kohler, PharmD, (Pharmacy Department); David Henderson,
MD, Laura Lee, RN (CC Office of the Director); Laura Chisholm, RN, Priscilla
Rivera, RN (Nursing Department); Linda Berry, RN, Greg Hinson, RPh, Lillian
Butler, RN (Information Systems Department); Frank Balis, MD, Greg Curt, MD,
and Wyndham Wilson, MD, PhD (NCI).
Parking renewals
NIH general parking permits
for campus employees whose last names begin with M or N will expire on the last
day of July. To renew yours, visit the NIH Parking Office in Bldg. 31, Room
B3B04, weekdays between 7:30 a.m. and 4:30 p.m. Be sure to bring your valid
NIH identification card, driver's license, and valid vehicle registration certificate
for each vehicle to be registered.
Slogan contest
You have until Sept. 1 to
enter the annual contest to find the perfect slogan for NIH Fire Safety Awareness
Day. The winning slogan and the author's name will appear on next year's official
NIH Fire Safety Awareness Day posters. Prizes will be awarded for the best qualifying
fire prevention slogans based on originality, inventiveness, and creativity.
For more information, call 6-0487. .
Construction
CRC construction status reports
and photos of progress are posted online monthly.
Volunteers needed
The Behavioral Endocrinology
Branch, NIMH, needs participants for two studies:
- Volunteers between the
ages of 18 and 45 are needed for a five-month study investigating the effects
of reproductive hormones on brain and behavior. Volunteers must be free of
medical illnesses and not taking any medication on a regular basis. For further
information, call Linda Simpson-St.Clair at 6-9576.
- Individuals with current
or past depression are needed to participate in an evaluation study at the
CC. These are not treatment studies for depression. Those eligible will receive
a physical evaluation, metabolic studies, and will participate in studies
for possible heart disease and osteoporosis in depression. Participants should
be 18-65 years old; otherwise healthy; non-smokers for the last year; and
able to spend at least one night at the CC. For details, call 6-5831 or 6-1892.
 Science
in the Cinema continues Science
in the Cinema continues
After the screening of each
film in this month's
Science in the Cinema, a guest speaker with expertise in the film's subject
area will comment on the science depicted in the film and take questions from
the audience. This Thursday evening program runs from 7 p.m. to about 9:30 p.m.
The Aug. 24 film will begin at 6:30 p.m. Come to Bldg. 45 (Natcher) auditorium.
Seating is first-come, first-served.
Aug. 10, 'Yellow Jack.' This
film is based on the true story of U.S. Army doctor Walter Reed (Lewis Stone)
and his quest to discover the cause of yellow fever (1938). Guest Speaker: Michael
Rhode, Chief Archivist, National Museum of Health and Medicine, Armed Forces
Institute of Pathology, Washington, D.C.
Aug. 17, 'Girl, Interrupted.'
Based on a true story, this film is set in the changing world of the late 1960s.
Finding herself at a renowned psychiatric institution for troubled women, Susanna
Kaysen must choose between the world of people who belong on the inside or the
often difficult world of reality on the outside (1999, Rated R). Guest Speaker:
Danny Wedding, PhD, MPH, Professor of Psychiatry and Director, Missouri Institute
of Mental Health, University of Missouri School of Medicine.
Aug. 24, 'Not As A Stranger.'
The story of a nurse who works to put her husband through medical school, this
film is a timeless depiction of the relational, financial, and emotional challenges
faced by those who embrace the medical profession (1955). Guest Speaker: Peter
Dans, MD, Associate Professor of Medicine, Department of Internal Medicine,
John Hopkins University School of Medicine.
For details, go to the Office
of Science Education web site at
http://science-education.nih.gov/cinema.
|
| Acting
Editor: Sara Byars |
Staff
Writers: Dina Dariotis, Bonnie Flock, LaTonya Kittles, Linda Silversmith
|
Clinical
Center News, 6100 Executive Blvd., Suite 3C01, MSC 7511, National Institutes
of Health, Bethesda, MD 20892-7511. Tel: 301-496-2563. Fax: 301-402-2984.
Published monthly for CC employees by the Office of Clinical Center Communications,
Colleen Henrichsen, chief. News, article ideas, calendar events, letters,
and photographs are welcome. Deadline for submissions is the second Monday
of each month.
|
|




 DTM
symposium set
DTM
symposium set Science
in the Cinema continues
Science
in the Cinema continues